What is a Standard Hydrogen Electrode
What is a Standard Hydrogen Electrode?
The Standard Hydrogen Electrode is regularly shortened to SHE, and its standard terminal potential is proclaimed to be 0 at a temperature of 298K. This is on the grounds that it goes about as a source of perspective for correlation with some other anode.
The redox half cell of the SHE is the place where the accompanying response happens:
2H+ (aq) + 2e–→ H2 (g)
The response given above by and large happens on a platinum anode. The weight of the hydrogen gas present in this half cell approaches 1 bar.
Utilization of Platinum in the Standard Hydrogen Electrode
Platinum is utilized in the Standard Hydrogen Electrode because of the accompanying reasons:
Platinum is a moderately dormant metal which doesn't consume without any problem.
Platinum has synergist characteristics which advances the proton decrease response.
The outside of platinum can be covered with platinum dark, a fine powder of platinum. This kind of platinum cathode is known as a platinized platinum terminal.
Platinum likewise improves the response energy by adsorbing hydrogen at the interface.
Standard Hydrogen Electrode Construction
The parts that make up a Standard Hydrogen Electrode are recorded underneath.
A platinum anode which is shrouded in finely powdered platinum dark (platinized platinum cathode).
A hydrogen Blow.
An answer of corrosive having a H+ molarity of 1 mole for every cubic decimeter.
The SHE likewise contains a hydroseal which is utilized to forestall the obstruction of oxygen.
The other half-cell of the whole Galvanic cell must be appended to the Standard Hydrogen Electrode through a repository to make an ionically conductive way. This should be possible through an immediate association, through a tight cylinder, or even using a salt scaffold.
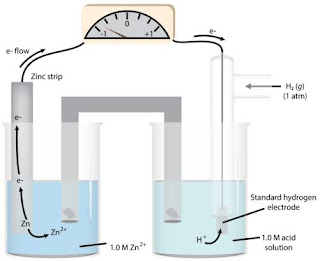
Standard Hydrogen Electrode Diagram
A named graph of a standard hydrogen cathode is given underneath. In the SHE represented, a salt extension is utilized to interface the SHE with the other half cell.
Standard Hydrogen Electrode Diagram
The platinized platinum surface has a high adsorption movement. Hence, this surface must be shielded from air oxygen just as from natural substances. Substances, for example, arsenic and sulfur mixes can deactivate or harm the impetus.
Every now and again Asked Questions – FAQs
What is the utilization of standard hydrogen cathode?
SHE is the fundamental guide for the revealing of the limit of quantitative half-cells. It is a sort of gas cathode and has been generally utilized as a source of perspective anode and as a pointer terminal for ascertaining pH esteems in early investigations.
What are the benefits of glass terminal?
There's a delicate glass cathode. It is utilized by numerous types of cell phones. Cleaning and alignment utilizing a typical cradle arrangement is simple and easy to utilize. It covers an acidic and an antacid pH range.
What is the standard hydrogen anode?
Ordinary hydrogen anode: This is a reference cathode to which all terminals are determined as far as cathode potential. On the off chance that hydrogen gas is adsorbed at 1 atm-pressure over a platinum anode dunked at 25oC in 1 M HCl, it is a customary cathode of hydrogen and its latent capacity is E0=±0 volt.
Comments
Post a Comment